

I WANT TO BE ON AN sHPT THERAPY THAT FITS MY NEEDS.
Have you been taken off Parsabiv® and are wondering why?
Find answersParsabiv® (etelcalcetide) is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with Read morechronic kidney disease (CKD) on hemodialysis.
Parsabiv® has not been studied in adult patients with parathyroid cancer, primary hyperparathyroidism, or with CKD who are not on hemodialysis and should not be used in these patients.
CloseYou are now leaving Parsabiv.com
Please know that the sponsors of this site are not responsible for content on the site you are about to enter.
Continue
You've come to the right place to learn how sHPT may affect you. Unmanaged sHPT can put your health at risk, but your care team is ready to help. This short video explains how to have this important dialogue about the treatment that's best for you.
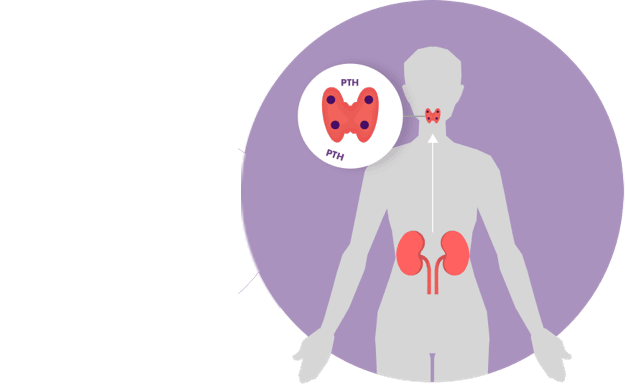

Some patients need medication to treat their sHPT. Parsabiv® is a treatment for sHPT in adult patients with chronic kidney disease on hemodialysis.


Want to take a deeper look into chronic kidney disease, sHPT, and ideas to help in your own care? Our resources are helpful for caregivers too.
Do not use Parsabiv® (etelcalcetide) if you are allergic to etelcalcetide or any ingredients in Parsabiv®. Allergic reactions, sometimes severe, have happened. Allergic reactions may include itchy rash, hives, swelling of the face, trouble breathing, and low blood pressure.
Do not use Parsabiv® (etelcalcetide) if you are allergic to etelcalcetide or any ingredients in Parsabiv®. Allergic reactions, sometimes severe, have happened. Allergic reactions may include itchy rash, hives, swelling of the face, trouble breathing, and low blood pressure.
Low calcium levels: Parsabiv® lowers calcium and can lead to low calcium levels in your blood, sometimes severe. Tell your healthcare provider if you have spasms, twitches, or cramps in your muscles; numbness or tingling in your fingers, toes, or around your mouth; or seizures. Low calcium levels can result in abnormal heart rhythms, known as ventricular arrhythmia. Tell your healthcare provider if you experience unusually fast or pounding heartbeat, if you have or have had heart rhythm problems or heart failure or if you take medicines that can cause heart rhythm problems while receiving Parsabiv®.
Very low calcium levels may increase the possibility of a seizure. Before starting Parsabiv®, tell your healthcare provider if you are taking medication to prevent seizures or have had seizures in the past. Report any seizure episodes while on Parsabiv®.
Do not take Parsabiv® with Sensipar® (cinacalcet) as severe, life-threatening low calcium levels can happen. Tell your healthcare provider if you are taking Sensipar®. When switching from Sensipar® to Parsabiv®, you should stop taking Sensipar® for at least 7 days before starting Parsabiv®.
Your healthcare provider will measure your blood calcium levels before starting and while being treated with Parsabiv®. Parsabiv® should not be started if your calcium levels are too low. Your healthcare provider will be able to tell you if your calcium is too low. While on Parsabiv®, your healthcare provider should perform repeated blood tests to monitor calcium and intact parathyroid hormone (iPTH) levels.
Worsening Heart Failure: Low blood pressure, heart failure, and decreased heart function have happened with Parsabiv®.
Upper Gastrointestinal Bleeding: In medical studies, 2 patients treated with Parsabiv® had upper gastrointestinal (GI) bleeding at the time of death. The exact cause of GI bleeding is unknown and there were too few cases to determine whether these cases were related to Parsabiv®.
Tell your healthcare provider if you have stomach pain, bloody or black stool, or if you vomit bloody or black material. Also tell your healthcare provider if you have nausea or vomiting that is getting worse.
Adynamic Bone Disease: Very low levels of PTH should be avoided to help maintain bone health.
Side Effects: The most common side effects of Parsabiv® are low calcium levels, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, and numbness or tingling in the fingers, toes, or around the mouth.
These are not all the possible side effects of Parsabiv®. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call
1-800-FDA-1088.
Indication Parsabiv® is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis.
Parsabiv® has not been studied in adult patients with parathyroid cancer, primary hyperparathyroidism, or with CKD who are not on hemodialysis and should not be used in these patients.
Please see accompanying Parsabiv® full Prescribing Information.